Bio-Containment Facilities
Bio-safety Laboratories (BSL-II / BSL-III / BSL-IV)
(As per WHO/ BMBL/ European Standards)
- Pre-fabricated (Modular) Bio-safety Laboratory
- Lab-in- Lab – Bio-safety Laboratory
- Construction type Bio-safety Laboratory
- Mobile Bio-safety Laboratory


—————————————————————————————————————————————————————————–
Plant Containment Laboratories (PCL-2/ PCL-3)
(As per WHO/ BMBL/ European Standards)
Plant Containment facilities is the containment level for plant pests. All Plant Containment physical and operational requirements apply to this containment level. Containment is achieved through the use of highly specialized facilities, stringent operational procedures and specialized equipment. Designing, constructing and maintaining a Plant Containment facility. The site for a containment facility should include an assessment of local agricultural and forestry programs as well as the local environment.
—————————————————————————————————————————————————————————–
Arthropod Containment Laboratories (ACL-2/ ACL-3)
(As per WHO/ BMBL/ European Standards)
Arthropod containment facility involves practices suitable for work with potential or known vectors that are, or may be infected with, BSL-3 agents associated with human disease. Arthropods that are infected or potentially infected with BSL-3 pathogens may pose an additional hazard if the insectary is located in an area where the species is indigenous, or if alternative suitable vectors are present, as an escaped arthropod may introduce the pathogen into the local population. Arthropod containment facility builds upon the practices, procedures, containment equipment, and facility requirements.
The BMBL states that “All procedures involving the manipulation of infectious materials are conducted within biological safety cabinets or other physical containment devices, or by personnel wearing appropriate protective clothing and equipment.”
—————————————————————————————————————————————————————————–
Modular Operating Theatre (Modular OT)
The Pre-fabricated Modular Operating Theatre offers Jointless uniform Sealed Non-Porus cleanable Surface.
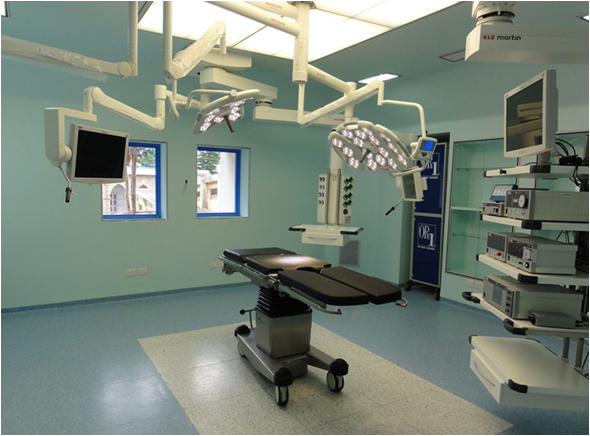
Controlled Environment, Uniform Air & light distribution Avoids Bacteria Contamination and prevent micro organism growth. Advantages of speed of construction and Maintenance. Allows Future expansion and Modernizati on in line with developments in surgical techniques.
—————————————————————————————————————————————————————————–
Clean Room Facility
Clean Room Technology is a critical aspect for a Pharmaceutical or Biotechnology facility. Elements contributing to a Clean Room are crucial to be considered right from conceptual stage a new set up.

Classified areas, air locks, pressure differentials go hand-in-hand with humidity, microbial load, temperature, in your facility. These issues are product and process dependant. Designing, fabrication and construction, installation and qualification of theses area all fall under one single aspect of Clean Room Facility and we provide these solutions
—————————————————————————————————————————————————————————–





